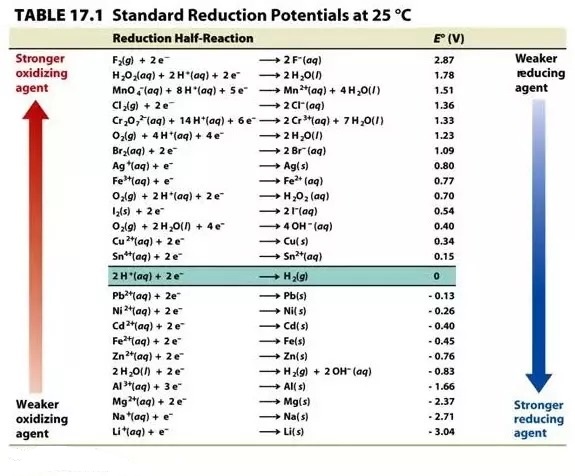
It is widely accepted that hydrogen has zero reduction and oxidation potentials. As a result, the difference in potential from hydrogen is actually used to calculate the standard reduction and oxidation potential of chemical species.
A voltmeter can be used to measure the potential difference from hydrogen in a galvanic cell that has a half-cell of the unidentified chemical species on one side and a SHE on the other.
The unidentified chemical species is reduced while hydrogen is oxidised when the standard reduction potential is calculated, and the unidentified chemical species is oxidised while hydrogen is reduced when the standard reduction potential table is calculated.
Read more:- https://mmsphyschem.com/estandardtable.htm
02/06/2023